|
 A New Tool to Detect Kidney Disease Outside the Hospital A New Tool to Detect Kidney Disease Outside the Hospital
|
|
• Fingerstick Capillary Blood Test
• As Easy to Use as Self-Testing for Blood Glucose
• Allows Screening or Monitoring of Kidney Disease
• Based on Nova’s Proven Hospital Creatinine/eGFR Meter
Nova Max Creatinine/eGFR is an important new tool to
improve kidney care through detection of kidney disease
early enough to successfully treat the disease. Nova Max
is a fast, accurate and easy to use meter and biosensor
for kidney function testing. The measurement technique
is very easy to use, virtually identical to the use of a
glucose meter by people with diabetes. Creatinine and
estimated glomerular filtration rate (eGFR) results are
reported (with or without race as a factor) using the
CKD-EPI equation from a small, capillary fingerstick
blood sample in just 30 seconds. Test results can be
wirelessly communicated to Bluetooth enabled applications
for review and intervention by healthcare
professionals.
Accuracy
Nova’s FDA cleared StatSensor Creatinine enzymatic technology has been used in hospital point-of-care meters for over 15 years. In a 2021 university medical center study of 670 patients sponsored by the International Society of Nephrology, Nova’s sensor technology proved more accurate than the laboratory Jaffe Creatinine/eGFR method when both methods were compared to the gold standard measured GFR (mGFR)

|
Very Good Correlation to Hospital Lab Analyzer
In a study performed for regulatory submission, 517 patients at three different sites had Nova Max Creatinine eGFR tests performed on fingerstick capillary blood samples. At the same time a venous blood sample was drawn and a laboratory analysis of creatinine/eGFR was performed. The creatinine method correlation was: 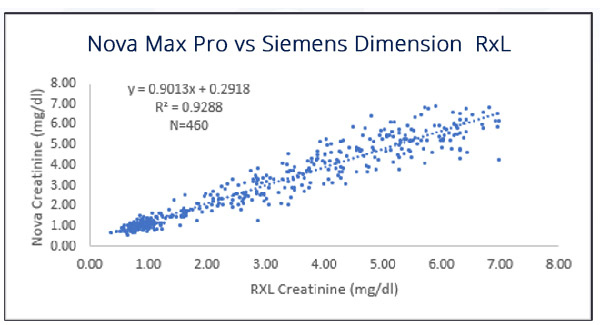
Very Good Sensitivity and Specificity
Sensitivity is the ability of a method to detect patients with the disease and specificity is the ability to detect patients without the disease.
Patients with an eGFR>60 ml/min/1.73m2 are considered to have normal kidney function, patients with eGFR<60 ml/min/1.73m2 are considered to have kidney disease. Nova Max uses the CKD-EPI equation to calculate eGFR. The results from this 517-patient study were:
| Sensitivity 98.9% (357/361) |
| Specificity 85% (133/156) |
| True Positives = 357 |
False Positives = 23 |
| True Negatives = 133 |
False Negatives = 4 |
Very Good Method Precision
Creatinine within-run and day-to-day precision studies were performed at three creatinine levels. 120 replicates of whole blood were used to calculate within-run precision and 240 replicates of controls were used to calculate day-to-day precision. The creatinine SD and CV%
are shown below:
| Day-to-Day Precision, Controls |
| |
Level 1 |
Level 2 |
Level 3 |
| N |
240 |
240 |
240 |
| Mean (mg/dL) |
1.5 |
3.4 |
5.5 |
| SD/CV% |
0.13 |
5.3% |
4.5% |
| Within Run Precision, Whole Blood |
| |
Level 1 |
Level 2 |
Level 3 |
| N |
120 |
120 |
120 |
| Mean (mg/dL) |
1.5 |
3.4 |
5.5 |
| SD/CV% |
0.08 |
5.4% |
4.0% |
Simple Test Procedure
 As Easy as Glucose Self-Testing Performed by People with Diabetes As Easy as Glucose Self-Testing Performed by People with Diabetes
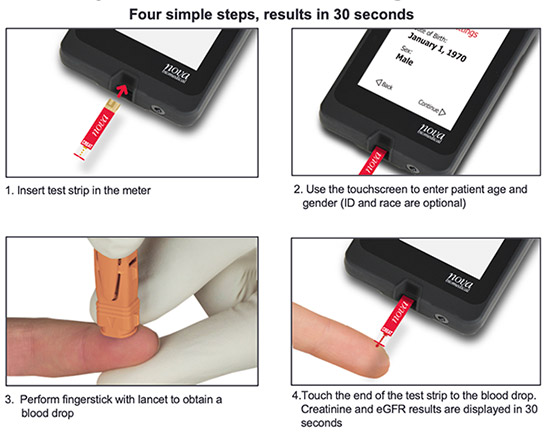
Capillary Blood Testing Eliminates a Venous Blood Draw
Capillary blood testing eliminates the need for a venipuncture by a phlebotomist, blood drawing tubes and needles.
Small Blood Sample
Nova Max Creatinine/eGFR capillary blood testing requires a 1.2 microliter finger prick sample and is virtually painless for the patient.
Other Features
 CKD-EPI 2021 eGFR Equation Is Used CKD-EPI 2021 eGFR Equation Is Used
This is the preferred method for estimation of glomerular filtration rate because of better accuracy at detecting early stages of kidney disease. The equation is offered with or without race as a factor.
Wireless Bluetooth Communication
Nova Max Creatinine/eGFR connects patient kidney data from the meter with healthcare professionals, medical records, or other applications
Bright, Color Touchscreen
The color touchscreen simplifies entry of patient age and sex. Abnormal test results are flagged with symbols and color.
Data Storage
Nova Max Pro stores up to 400 patient and quality control results. Results are easily recalled for review or data transmission.

Current Field Studies
Nova Max Pro Creatinine measuring technology is based on the Nova StatSensor Creatinine technology which has been used in point-of-care applications for over 15 years. Nova’s technology has been proven in numerous non-hospital settings, including pharmacies, community health centers, imaging centers, clinics, physician offices, and home testing.
Pharmacies
 A recent study highlight-ed the value of eGFR
testing in community
pharmacies in Spain.6 The Nova creatinine
eGFR meter was used
to evaluate patients that
were asymptomatic for CKD but were either on potentially
nephrotoxic medications or at risk for chronic kidney
disease. Almost 200 patients were tested in four pharmacies,
with 44% of them showing an eGFR of <60 (the level
indicating kidney disease). Of these patients with a low eGFR, almost half of them (43%) had medication dosages that
required adjustment or discontinuation. This study highlights
the value of eGFR testing in the outpatient setting, and the
important patient improvements it can bring. The study is
being expanded to 5,000 patients.
Another pharmacy study in the Netherlands used Nova
Creatinine/eGFR to screen
asymptomatic CKD patients with
previously unknown kidney disease who were prescribed non-steroidal
anti-inflammatory drugs. The authors found that “POC measurement of creatinine with eGFR estimation changed
the prescription of NSAIDs in almost 25% of patients with previously unknown renal function.”7 A recent study highlight-ed the value of eGFR
testing in community
pharmacies in Spain.6 The Nova creatinine
eGFR meter was used
to evaluate patients that
were asymptomatic for CKD but were either on potentially
nephrotoxic medications or at risk for chronic kidney
disease. Almost 200 patients were tested in four pharmacies,
with 44% of them showing an eGFR of <60 (the level
indicating kidney disease). Of these patients with a low eGFR, almost half of them (43%) had medication dosages that
required adjustment or discontinuation. This study highlights
the value of eGFR testing in the outpatient setting, and the
important patient improvements it can bring. The study is
being expanded to 5,000 patients.
Another pharmacy study in the Netherlands used Nova
Creatinine/eGFR to screen
asymptomatic CKD patients with
previously unknown kidney disease who were prescribed non-steroidal
anti-inflammatory drugs. The authors found that “POC measurement of creatinine with eGFR estimation changed
the prescription of NSAIDs in almost 25% of patients with previously unknown renal function.”7
Community Screening of At-Risk Individuals

Nova Creatinine/eGFR measuring technology has proven to be an excellent tool for kidney screening and early detection of kidney disease in community health centers. One study concluded: “We effectively utilized a clinical symptom-based score and deployed POC creatinine and urine dipstick tests for screening and identification of patients with kidney dysfunction across three different low-resource settings. We described a practical approach for evaluating and classifying patients as AKD, CKD, or NKD in the absence of knowledge of their prior state of kidney health, to guide further evaluation and follow-up.”3
Nova POC devices have also been used to assess kidney function in community healthcare clinics in Low and Middle- Income Countries (LMIC) to screen for patients at risk for kidney disease.4, 5 Even in wealthier countries, individuals living in remote areas face obstacles in terms of access to routine laboratory testing, and Nova Max eGFR can be a cost-effective solution to allow for screening and monitoring of these populations. Importantly, there is no sacrificing quality and accuracy using the Nova device to evaluate kidney disease, as a head-to-head comparison with the Nova eGFR device showed it to be more accurate than a hospital laboratory analyzer when compared to a true measured GFR.4
Clinics and Physician Offices

AstraZeneca has begun
a study using Nova Max
Creatinine/eGFR in
hundreds of physician
offices in 25 countries
to screen asymptomatic
but potentially at-risk
patients for kidney disease. As with other screening studies, the thesis is that early detection will improve outcomes for patients by allowing early treatment.
Imaging Centers
 Administering contrast
dyes for radiologic
exams is known to be
potentially nephrotoxic,
making kidney function
assessment recommended
prior to administering contrast media. Since many of these studies take place in outpatient imaging facilities, patients without recent eGFR test results may face cancellation of their exam or delays.
Several studies have now evaluated the use of Nova
Biomedical devices for testing in these facilities, with
results that show acceptable accuracy and error rates when
compared with central laboratory analyzers.8-14
In the case of procedures on patients at high risk for contrast-induced nephropathy, such as cardiac catheterization and peripheral arteriography, contrast loads can be high, and kidney injury may not be apparent for several days after the procedure. In these cases, patients could be sent home with the device to allow early detection of kidney injury. Administering contrast
dyes for radiologic
exams is known to be
potentially nephrotoxic,
making kidney function
assessment recommended
prior to administering contrast media. Since many of these studies take place in outpatient imaging facilities, patients without recent eGFR test results may face cancellation of their exam or delays.
Several studies have now evaluated the use of Nova
Biomedical devices for testing in these facilities, with
results that show acceptable accuracy and error rates when
compared with central laboratory analyzers.8-14
In the case of procedures on patients at high risk for contrast-induced nephropathy, such as cardiac catheterization and peripheral arteriography, contrast loads can be high, and kidney injury may not be apparent for several days after the procedure. In these cases, patients could be sent home with the device to allow early detection of kidney injury.
Home Testing
There are numerous opportunities for patients to benefit from eGFR self-testing. Monitoring of eGFR at home provides
convenience, cost-effectiveness, and improved outcomes
by early detection of kidney disease. Populations that have
been studied using the Nova meter at home include
cardiac catheterization patients (unpublished data from ongoing study), renal transplant patients, and Native
American populations living in remote settings.15-19
Ongoing home self-testing studies with the Nova meter are being conducted by the Chronic Renal Insufficiency Cohort (CRIC)* for monitoring patients with kidney disease.20
Home Testing for Kidney Transplant Patients
After kidney transplantation, early detection of transplant
failure is mandatory to minimize harm to the patient and
permanent damage to the transplanted organ. Patients therefore have laboratory eGFR/creatinine tests on average 20 times during the first year post transplantation. The high frequency of outpatient visits after kidney transplantation is burdensome to the recovering patient and to health care
system capacity. A self-monitoring program can improve
post transplantation care by early identification of acute rejection due to higher frequency of testing, decrease the high number of outpatient visits, increase patient
satisfaction, all of which may improve kidney-graft
survival. A study is beginning at the UK National Health
Service (NHS) to monitor kidney transplant patients at home by having their self-test results transmitted automatically
to clinical staff for evaluation and intervention.
Kidney Disease is a Worldwide Healthcare Crisis
Kidney disease (CKD) is a major healthcare crisis. It is growing at an accelerated rate, rising from 13th to 10th place in the World Health Organization ranking of most common
causes of death. It currently represents a huge healthcare economic burden in every country and because of its growth it will be an even larger one in the future unless CKD can be detected and treated early.
If Detected Early, Kidney Disease Progression can be Prevented or Delayed 1
As many as 90% of people with kidney disease are
undiagnosed.1 In the U.S. alone 15 million adults are
estimated to have CKD but 13.5 million are unaware they have it. Kidney disease is often termed the “silent killer” because it shows no symptoms until it is very late stage when there are few treatment options other than
dialysis, a very difficult end-stage treatment for patients and very expensive for the healthcare system. Because
kidney disease is almost always detected too late, treatment options are still associated with high mortality. Today, the availability of the first drug to retard the progression of
kidney disease (Dapagliflozin/Farxiga, Astra Zeneca) makes it even more important to detect kidney disease
early so treatment can be started before it is too late.2
Other novel therapeutics are in various stages of development and also show potential to treat kidney disease.3
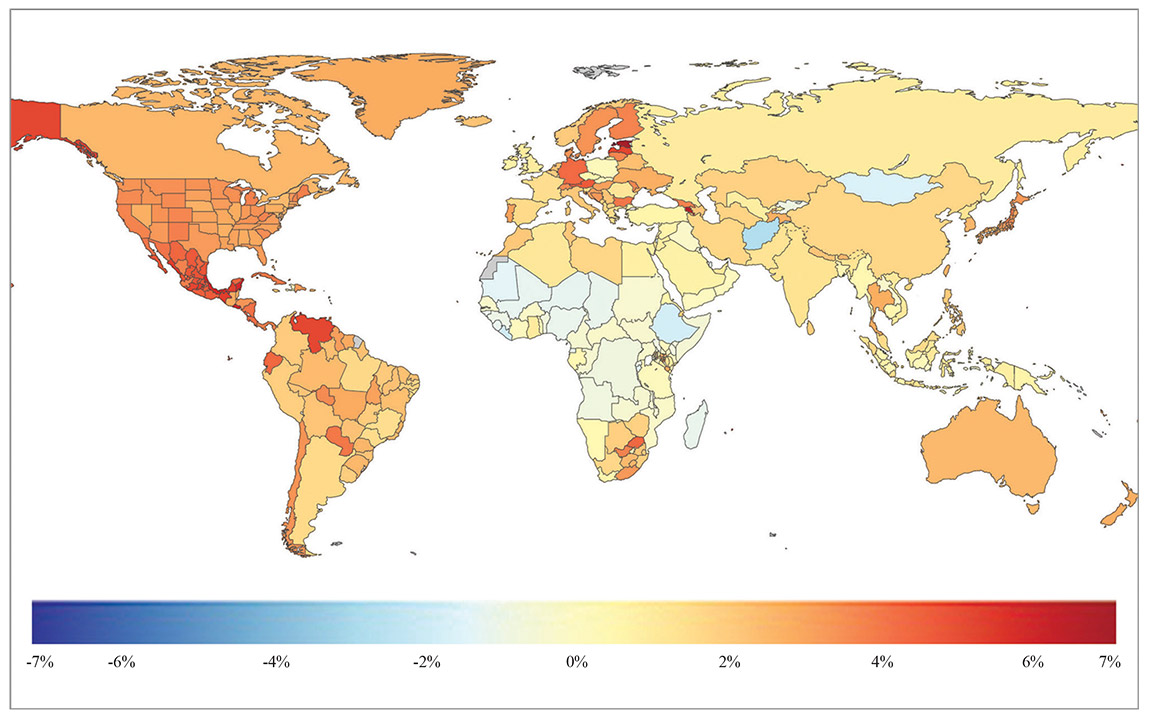 Global Map of Chronic Kidney Disease, Both sexes, all ages, annual % change, 1990 to 2019, deaths per 100,000 Global Map of Chronic Kidney Disease, Both sexes, all ages, annual % change, 1990 to 2019, deaths per 100,000
Nova Max Pro Specifications
Test Measured: Creatinine
Tests Reported: Creatinine, eGFR
Test Time: 30 seconds
Test Strip Volume: 1.2 µL
Test Methodology: Electrochemistry
Weight: 90g (3.17 oz)
Size: 3.75 in x 2.44 in x 0.74 in
(95.25 mm x 61.98 mm x 18.80 mm)
Sample Types and Operating Modes:
Whole Blood: Capillary
Measurement Range:
Creatinine: 0.30-7.00 mg/dL or 27-619 μmol/L
Operating Ranges:
Temperature: 59˚F – 104˚F (15˚C – 40˚C)
Altitude: 15,000 feet (Up to 4,500 meters)
Humidity: 10% to 90% relative humidity
Test Strips and Reagents:
Test Strip Refrigerated Storage: 12 months 36˚F – 46˚F (2˚C – 8˚C)
Test Strip In-Use
Stability: 3 months
Quality Control 3 levels (low, normal, high)
Data Storage:
Patient and QC Tests: 400
eGFR:
CKD-EPI2021
FDA Labeling:
For in-vitro diagnostic use
Connectivity:
Meter Data Output: Wireless Bluetooth connectivity
Battery Information:
Type: 3.7V Li Polymer Rechargeable Battery
Additional Features:
• LCD large color display • Traditional QC with target values assigned to QC materials • Units of measure based on meter (mg/dL or µmol/L) • Automatic shut-off when not in use • Automatic sample detection and analysis • Automatic sample counting with date/time stamp for data tracking
Optional Carry Case:
Robust plastic storage/carry case holds meter, test strips, controls vials, lancets, charging cord with plug, quick reference guide, and removable meter stand.
Certifications and Compliance:
ISO 13485:2016 Quality System Registration, IVDD, CE Self Declared,
Tested according to: EN 61010-1:2010, EN 61010-2-101:2015
|
REFERENCES
1.Foundation NK. Kidney Disease: The Basics. Details here
2.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjostrom CD, Toto RD, Langkilde AM, Wheeler DC, Committees D-CT and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446.
3.Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL, FIDELIO-DKD obot and investigators F-D. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. European heart journal. 2021.
4.Currin S, Gondwe M, Mayindi N, Chipungu S, Khoza B, Khambule L, Snyman T, Tollman S, Fabian J, George J and Consortium ARK. Evaluating chronic kidney disease in rural South Africa: comparing estimated glomerular filtration rate using point-of-care creatinine to iohexol measured GFR. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2021.
5.Macedo E, Hemmila U, Sharma SK, Claure-Del Granado R, Mzinganjira H, Burdmann EA, Cerda J, Feehally J, Finkelstein F, Garcia-Garcia G, Jha V, Lameire NH, Lee E, Levin NW, Lewington A, Lombardi R, Rocco MV, Aronoff-Spencer E, Tonelli M, Yeates K, Remuzzi G, Mehta RL and Group ISNbTS. Recognition and management of community-acquired acute kidney injury in low-resource settings in the ISN 0by25 trial: A multi-country feasibility study. PloS Medicine. 2021;18:e1003408.
6.Iker Cámara-Ramos GE-M, Mª Teresa Climent-Catalá, Luis Salar-Ibáñez THE IMPORTANCE OF COMMUNITY PHARMACY IN CHRONIC KIDNEY DISEASE PATIENT MANAGEMENT. DRUG DOSAGE ADJUSTMENT AND NEPHROTOXICITY DETECTION. Poster-WONCA. 2021.
7.Blairon L, Abbasi M, Beukinga I, Melot C and Libertalis M. Improving NSAIDs Prescription in Emergency Services Unit by a Point-of-Care-Based Renal Function Evaluation. The Journal of emergency medicine. 2020;58:481-486.
8.Mathur N, Lu ZX, MacKay L, Lau T, Kuganesan A and Lau KK. Is point of care renal function testing reliable screening pre-IV contrast administration? Emerg Radiol. 2021;28:77-82.
9.Inoue A, Nitta N, Ohta S, Imoto K, Yamasaki M, Ikeda M and Murata K. StatSensor-i point-of-care creatinine analyzer may identify patients at high-risk of contrast-induced nephropathy. Experimental and therapeutic medicine. 2017;13:3503-3508.
10.Wibmer A, Nolz R, Heinz-Peer G, Wien/AT and Polten/AT S. Rapid bedside assessment of the renal function of patients undergoing contrast-enhanced CT. Is it a reliable approach for identifying patients at risk of a contrast medium adverse reaction? ECR 2013. 2013;Poster no. C-2669:1-9.
11.Lee-Lewandrowski E, Chang C, Gregory K and Lewandrowski K. Evaluation of rapid point-of-care creatinine testing in the radiology service of a large academic medical center: impact on clinical operations and patient disposition. Clinica chimica acta; international journal of clinical chemistry. 2012;413:88-92.
12.Snaith B, Harris MA, Shinkins B, Jordaan M, Messenger M and Lewington A. Point-of-care creatinine testing for kidney function measurement prior to contrast-enhanced diagnostic imaging: evaluation of the performance of three systems for clinical utility. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2018;56:1269-1276.
13.Corbett M, Duarte A, Llewellyn A, Altunkaya J, Harden M, Harris M, Walker S, Palmer S, Dias S and Soares M. Point-of-care creatinine tests to assess kidney function for outpatients requiring contrast-enhanced CT imaging: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-248.
14.Vilaine E, Gabarre P, Beauchet A, Seidowsky A, Auzel O, Moreau MH, Dubourg O, Mansencal N, Essig M and Massy Z. Point-of-Care Capillary Blood Creatinine: A Prospective Study In Cardiology and Nephrology Outpatients. 2020.
15.Nataatmadja M, Fung AWS, Jacobson B, Ferera J, Bernstein E, Komenda P, Mattman A, Seccombe D and Levin A. Performance of StatSensor Point-of-Care Device for Measuring Creatinine in Patients With Chronic Kidney Disease and Postkidney Transplantation. Canadian Journal of Kidney Health and Disease. 2020;7:2054358120970716.
16.van Lint CL, van der Boog PJ, Wang W, Brinkman WP, Rovekamp TJ, Neerincx MA, Rabelink TJ and van Dijk S. Patient experiences with self-monitoring renal function after renal transplantation: results from a single-center prospective pilot study. Patient Prefer Adherence. 2015;9:1721-31.
17.van Lint CL, van der Boog PJ, Romijn FP, Schenk PW, van Dijk S, Rovekamp TJ, Kessler A, Siekmann L, Rabelink TJ and Cobbaert CM. Application of a point of care creatinine device for trend monitoring in kidney transplant patients: fit for purpose? Clinical chemistry and laboratory medicine : CCLM / FESCC. 2015;53:1547-56.
18.Boesten LSM and Pelt Jv. Clinical evaluation of a point of care device for creatinine measurements in finger prick blood for the follow-up of kidney transplant patients. 2010.
19.Unruh ML, Arzhan S, Feldman HI, Looker HC, Nelson RG, Faber T, Johnson D, Son-Stone L, Pankratz VS, Myaskovsky L and Shah VO. American Indian chronic Renal insufficiency cohort study (AI-CRIC study). BMC nephrology. 2020;21:291.
20.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu C-y, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, Muntner P, Ojo AO, Rahman M, Townsend RR and Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology. 2003;14:S148-S153.
 A New Tool to Detect Kidney Disease Outside the Hospital
A New Tool to Detect Kidney Disease Outside the Hospital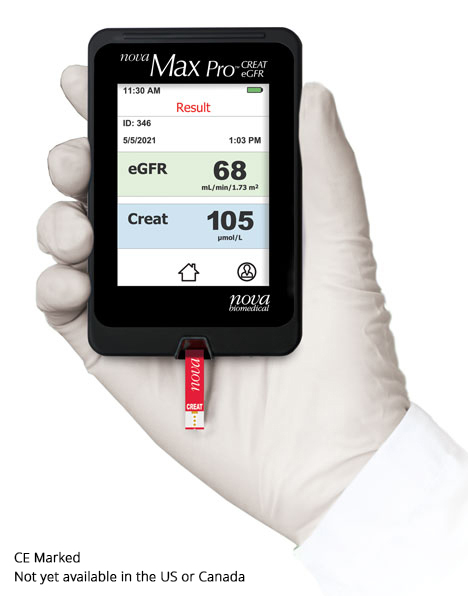







 A recent study highlight-ed the value of eGFR
testing in community
pharmacies in Spain.6 The Nova creatinine
eGFR meter was used
to evaluate patients that
were asymptomatic for CKD but were either on potentially
nephrotoxic medications or at risk for chronic kidney
disease. Almost 200 patients were tested in four pharmacies,
with 44% of them showing an eGFR of <60 (the level
indicating kidney disease). Of these patients with a low eGFR, almost half of them (43%) had medication dosages that
required adjustment or discontinuation. This study highlights
the value of eGFR testing in the outpatient setting, and the
important patient improvements it can bring. The study is
being expanded to 5,000 patients.
Another pharmacy study in the Netherlands used Nova
Creatinine/eGFR to screen
asymptomatic CKD patients with
previously unknown kidney disease who were prescribed non-steroidal
anti-inflammatory drugs. The authors found that “POC measurement of creatinine with eGFR estimation changed
the prescription of NSAIDs in almost 25% of patients with previously unknown renal function.”7
A recent study highlight-ed the value of eGFR
testing in community
pharmacies in Spain.6 The Nova creatinine
eGFR meter was used
to evaluate patients that
were asymptomatic for CKD but were either on potentially
nephrotoxic medications or at risk for chronic kidney
disease. Almost 200 patients were tested in four pharmacies,
with 44% of them showing an eGFR of <60 (the level
indicating kidney disease). Of these patients with a low eGFR, almost half of them (43%) had medication dosages that
required adjustment or discontinuation. This study highlights
the value of eGFR testing in the outpatient setting, and the
important patient improvements it can bring. The study is
being expanded to 5,000 patients.
Another pharmacy study in the Netherlands used Nova
Creatinine/eGFR to screen
asymptomatic CKD patients with
previously unknown kidney disease who were prescribed non-steroidal
anti-inflammatory drugs. The authors found that “POC measurement of creatinine with eGFR estimation changed
the prescription of NSAIDs in almost 25% of patients with previously unknown renal function.”7 

 Administering contrast
dyes for radiologic
exams is known to be
potentially nephrotoxic,
making kidney function
assessment recommended
prior to administering contrast media. Since many of these studies take place in outpatient imaging facilities, patients without recent eGFR test results may face cancellation of their exam or delays.
Several studies have now evaluated the use of Nova
Biomedical devices for testing in these facilities, with
results that show acceptable accuracy and error rates when
compared with central laboratory analyzers.8-14
In the case of procedures on patients at high risk for contrast-induced nephropathy, such as cardiac catheterization and peripheral arteriography, contrast loads can be high, and kidney injury may not be apparent for several days after the procedure. In these cases, patients could be sent home with the device to allow early detection of kidney injury.
Administering contrast
dyes for radiologic
exams is known to be
potentially nephrotoxic,
making kidney function
assessment recommended
prior to administering contrast media. Since many of these studies take place in outpatient imaging facilities, patients without recent eGFR test results may face cancellation of their exam or delays.
Several studies have now evaluated the use of Nova
Biomedical devices for testing in these facilities, with
results that show acceptable accuracy and error rates when
compared with central laboratory analyzers.8-14
In the case of procedures on patients at high risk for contrast-induced nephropathy, such as cardiac catheterization and peripheral arteriography, contrast loads can be high, and kidney injury may not be apparent for several days after the procedure. In these cases, patients could be sent home with the device to allow early detection of kidney injury. Global Map of Chronic Kidney Disease, Both sexes, all ages, annual % change, 1990 to 2019, deaths per 100,000
Global Map of Chronic Kidney Disease, Both sexes, all ages, annual % change, 1990 to 2019, deaths per 100,000