 Critical Care Blood Gas Analyzer
Critical Care Blood Gas Analyzer
New Technologies Simplify Use and Offer Additional Tests
Stat Profile Prime Plus is a comprehensive, whole blood critical care analyzer that combines blood gases, electrolytes, metabolites, CO-Oximetry, and 34 calculated results in a simple, compact analyzer. Prime Plus combines maintenance-free, component cartridge technology for sensors and reagents with patented, new, maintenance-free, and non-lysing whole blood CO-Oximetry technology.
Prime Plus results are produced rapidly, a complete test menu panel in about 90 seconds, and are combined with bidirectional connectivity, a robust data management system, and comprehensive cybersecurity protection.
Nova MicroSensor Card™ Technology

Most comprehensive critical care menu
PO2 PCO2 pH Hct tHb Na Cl K iCa TCO2 iMg Glu Lac Urea (BUN) Creat SO2% O2Hb COHb MetHb HHb HbF tBil
- All Prime Plus biosensors use proven Nova technology in a miniaturized, maintenance-free sensor card format.
- Nova’s MicroSensor cards combine all 22 whole blood assays including CO-Oximetry.
Important New Assays
 Urea (BUN), Creatinine and eGFR
Urea (BUN), Creatinine and eGFR
Over 50% of patients admitted to the ICU will develop some stage of acute kidney injury (AKI).1 Stat Profile Prime Plus is the only blood gas analyzer to provide whole blood urea (BUN) and creatinine (plus eGFR) test options for rapid assessment of kidney function.
Ionized magnesium (iMg)
Disruptions in the balance of iMg, Na, K, and iCa can cause cardiac arrhythmias, reduced cardiac contraction, and cardiac arrest. Prime Plus is the only blood gas analyzer to provide a comprehensive profile of electrolytes including iMg.
Estimated Plasma Volume (ePV)
Prime Plus analyzers have the unique ability to report estimated plasma volume (ePV), a very important test that assesses the intravascular fluid content of blood, the patient’s hydration status. The plasma volume status of a patient is one of the top priorities in managing many different conditions including shock, sepsis, congestive heart failure, acute or chronic kidney disease, chronic pulmonary disease, as well as general postoperative care.
Prime Plus reports ePV and ΔPV using the Strauss equation which requires measured hemoglobin and measured hematocrit to determine ePV. ePV may be most beneficial when it is measured serially, and thus the change in plasma volume (ΔPV) can also be used to guide fluid therapy.
New Disposable CO-Oximetry Technology Eliminates Maintenance
Prime Plus incorporates a new, patented multi-wavelength optical system that scans a continuous spectrum of optical wavelengths to enable a comprehensive CO-Oximetry panel result without lysing the sample. The optical components in contact with blood are contained in the disposable sensor card.
- Cleaning and deproteinising are completely eliminated.
- Lysing and all its required mechanical components are eliminated, as are lysing and deproteinising reagents. This improves reliability and reduces maintenance and costs.
CO-Oximetry test menu
O2Hb COHb MetHb HHb tHb HbF* tBil*
Fast Stat Results
Prime Plus’s exceptional throughput easily handles the high sample workload of a busy critical care setting. Prime Plus delivers a 20-test critical care profile in about 90 seconds. Competitors’ analyzers can require up to four minutes, even with fewer tests reported.Clot Protection
Prime Plus’s unique Clot Block™ sample flow path protects sensor cards from blood clot blockages.Bidirectional Connectivity Patient Management
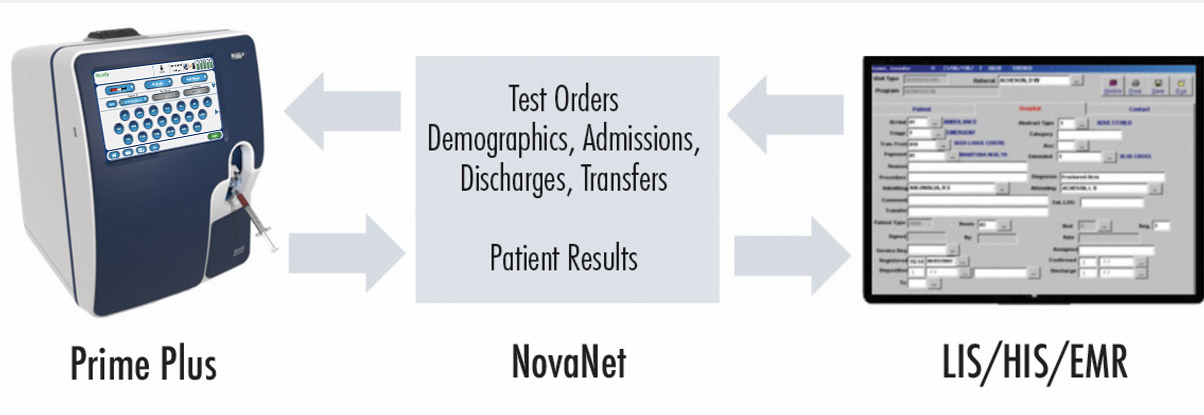
NovaNet bidirectional middleware for all Nova connected devices
NovaNet ensures timely, accurate capture of Nova analyzer test results for clinicians and managers to retrieve wherever and whenever needed. Also included is comprehensive cybersecurity protection and encryption that provides protection against attempts to access a hospital's network.
Automated, True Liquid QC
 Liquid QC provides the only reliable test of analyzer performance
Liquid QC provides the only reliable test of analyzer performance
United States federal government regulations and many international government regulations have eliminated electronic equivalent QC and are requiring true
liquid QC.2
Automated QC complies with U.S. CLIA, German RiLiBAK, and other international QC requirements
QC cartridges contain a 30-day supply of liquid QC material. Controls are run automatically at user-selected intervals. Prime Plus quality controls:
- Are comprised of a similar matrix to that of patient samples.
- Are treated in the same manner as patient samples.
- Follow the exact sample pathway as patient samples, from sample probe to waste container.
- Challenge all analytical phases of testing.
- Challenge testing at patient low, normal, and high value ranges.
Supplemental Quality Monitoring (SQM)
Prime Plus provides an automated electronic quality monitoring supplement to liquid QC. SQM continuously monitors the status and performance of all analytical components (including sensors, reagents, calibrators, sample integrity, software, and electronics), providing real-time, sample-to-sample assurance of correct performance.
For more product information, click here or contact Nova Biomedical at:
Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800
For Nova Biomedical Sales inquiries, reach us at 1-800-458-5813.
For Technical Support, reach us at 1-800-545-NOVA (6682).
To place an order, call 1-800-822-0911 or email novaorders@novabio.com
1. Mandelbaum T et al. Outcome of critically ill patients with acute kidney injury using the Akin criteria. Crit Care Med 2011;39:2259-2264.
2. Centers for Medicare and Medicard Services, Center for Clinical Standards and Quality/Survey and Certification Group. Policy clarification or acceptable control materials used when quality control (QC) is performed in laboratories. Baltimore, MD: CMS, April 8, 2016.
Stat Profile Prime Plus® Specifications
| Critical Care Test Menu | Methodology |
| pH | Direct ISE |
| PCO2 | Severinghaus |
| PO2 | Amperometric |
| SO2% | Optical, reflectance |
| Hematocrit | Conductivity/Na correction |
| Na | Direct ISE |
| K | Direct ISE |
| Cl | Direct ISE |
| iCa | Direct ISE |
| iMg | Direct ISE |
| Glucose | Enzyme/Amperometric |
| Lactate | Enzyme/Amperometric |
| Urea (BUN) | Enzyme/Amperometric |
| Creat | Enzyme/Amperometric |
Calculated Tests
| eGFR | A-aDO2 | ePV and delta PV |
| HCO3- | a/A | Normalized iCa |
| TCO2 | PO2/FIO2 | Normalized iMg |
| BE-ecf | Anion Gap | Osmolality |
| BE-b | SBC | Hemoglobin |
| A | Base Excess | O2 Saturation |
| ePV (Estimated Plasma Volume) | ||
| pH/PCO2/PO2 Corrected to Patient Temperature Respiratory Index (If % FIO2 value entered) Actual Bicarbonate Standard Bicarbonate Oxygen Index (OI) |
||
| CO-Oximetry Tests | |
| HHb, deoxyhemoglobin | O2Hb, oxyhemoglobin |
| MetHb, methemoglobin | COHb, carboxyhemoglobin |
| tHb, total hemoglobin | SO2%, oxygen saturation |
| tBil, total bilirubin | HbF, fetal hemoglobin |
Compact Size
Dimensions for Prime Plus, including CO-Oximetry and bidirectional connectivity:
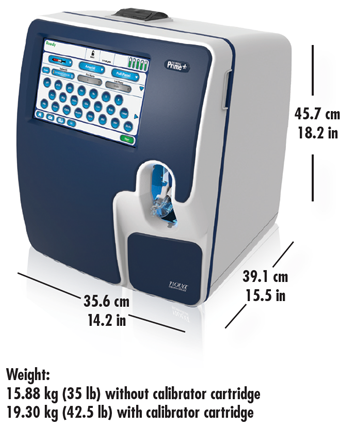
Other Features
Full color, 10.1-inch touchscreen, multilingual, QC statistics, onboard data management, automatic sampler, integrated capillary adapter, optional barcode scanner, QC data storage, optional mobile cart with UPS
MicroSensor Card
60 µL Sample Volume
Electrical Power Requirement
< 90 Watts
Acceptable Samples
Whole blood (heparinized), arterial, venous, mixed venous
135 µL Sample draw requirement
Communication Protocols
ASTM, HL7, or POCT01-A2 connectivity formats
Certifications and Compliance: Nova Biomedical is certified to FDA Quality System Regulations and EN ISO 13485:2016 Complies to IVDD Tested according to: EN 61010-1:2010, EN 61010-2-101:2015, EN 60825-1/A1:2014.
Specifications current as of revision date.